

When Delta H is negative, it’s regarded as an exothermic reaction. Furthermore, a negative Delta H means that the heat flows from a system to its surroundings. ĭelta H being negative suggests that the reaction gives off heat from reactants to products, which is considered favorable. What Does it Mean if Delta H is Positive (+) or Negative (-)?Īs mentioned above, a negative Delta H is associated with a decrease in net energy, and a positive Delta H indicates an increase in total power. In other words, you can find Delta H in chemistry by subtracting the sum of enthalpies of the reactants from the total enthalpies of the products. You must calculate the total mass of the reactants (m), the particular heat of the product (s), and Delta T, which is the temperature change from the reaction.īy simply plugging the values in the formula, we can multiply and solve for the change in enthalpy. To determine the change in enthalpy you must make calculations.

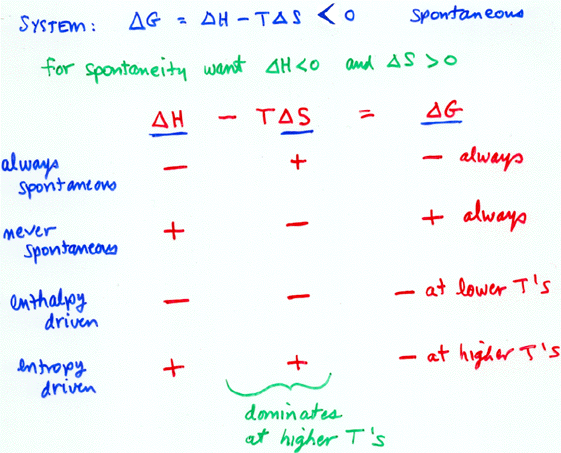
The formula for enthalpy or Delta H is ∆H = m x s x ∆T. On the other hand, if Delta H or enthalpy is negative, this is associated with a decrease in the total energy held within a system. Therefore, if the change in enthalpy or Delta H is positive, that indicates an increase in the total amount of power within the system. In contrast to entropy, enthalpy measures the total energy within a particular system. It is used to describe whether a system absorbs or emits heat. To understand Delta S better, let’s look at Delta H first. This is how you may remember the terms and differentiate between them more easily. An easier way to not forget this is to associate and remember the “H” present in Delta H and enthalpy.Īs enthalpy contains an H, it becomes easier to associate it with Delta H. As you have noticed, Delta H has “H” and does enthalpy.Īutomatically, this makes Delta S or entropy. Here is a tip that will help you remember the two terms better! Please take a look at their respective spelling. It is easy to mix up their meanings and use them in other contexts as they sound similar. However, I’ve found that people often confuse the two terms. What is the Difference Between Delta H and Delta S?ĭelta H and Delta S are different things altogether.What is the relationship between Delta S and Delta H?.Predicting if Delta S is Positive (+) or Negative (-)?.What Does a Positive or Negative Delta S Mean?.What Does Delta S Represent in Chemistry?.Examples for a Positive or Negative Delta H:.What Does it Mean if Delta H is Positive (+) or Negative (-)?.


 0 kommentar(er)
0 kommentar(er)
